University of Cologne Researchers Discover a Key Mechanism That Controls Human Heart Development
 © Deniz Bartsch
© Deniz Bartsch
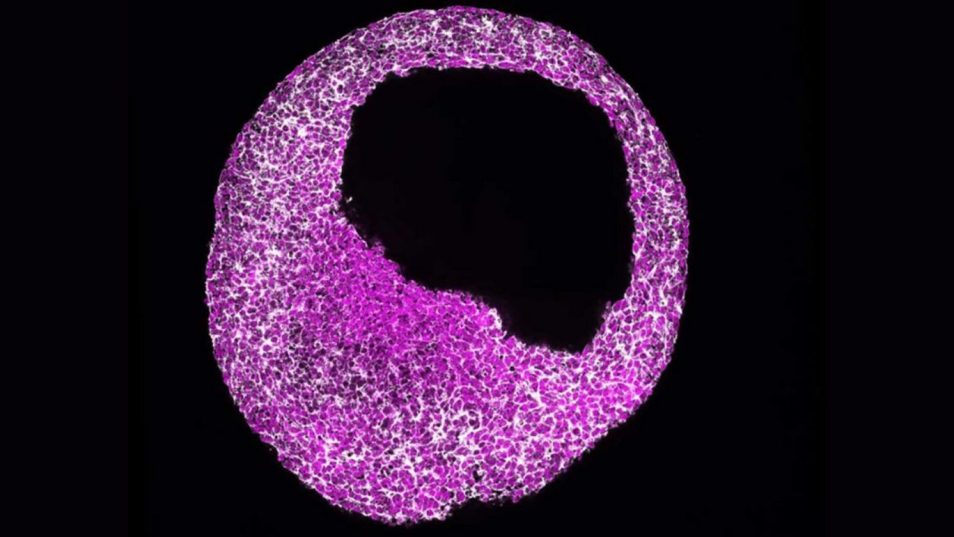
Writing in ‘Science Advances’ researchers of the University of Cologne describe a key mechanism that controls the decision-making process that allows human embryonic stem cells to make the heart. These discoveries enable better insights into how the human heart forms in an embryo and what can go wrong during heart formation, causing cardiac disease or, in the worst case, embryo termination.
In humans, a specialized mRNA translation circuit predetermines the competence for heart formation at an early stage of embryonic development, a research team at the Institut für Neurophysiology, the Center for Molecular Medicine Cologne (CMMC) and the University of Cologne’s Cluster of Excellence in Aging Research CECAD led by Junior Professor Dr Leo Kurian has discovered. While it is well known that cardiac development is prioritized at the early stages of embryogenesis, the regulatory programme that controls the prioritization of the development of the heart remained unclear until now. Kurian and his team investigated how the prioritization of heart development is regulated at the molecular level. They found that the protein RBPMS (RNA-binding protein with multiple splicing) is responsible for the decision to make the heart by programming mRNA translation to approve future cardiac fate choice. The study is published under the title ‘mRNA translational specialization by RBPMS presets the competence for cardiac commitment’ in Science Advances.
One out of 100 children born with a cardiac disease
A better understanding of human cardiac development is essential not only to determine the fundamental principles of self-organization of the human heart but also to reveal molecular targets for future therapeutic interventions for congenital and adult-onset cardiac disease.
Since the heart is the first functional organ to form in a developing embryo, any anomaly in early embryonic cell fate decisions needed for the development of the heart leads to catastrophic consequences, often resulting in the termination of pregnancy or lifelong suffering due to congenital heart diseases. In humans, approximately 30 percent of developing embryos terminate before implantation in the uterus, and about 25 percent fail during the transition from gastrulation (the early phase when the embryo begins to differentiate distinct cell lineages) to organogenesis (the phase that lasts until birth when all tissues and organs form and mature).
Often, the cause of embryo termination is impaired cardiovascular cell fate decisions and morphogenesis, the biological process by which a cell, a tissue or an organism develops its form. The failure to accurately specify cell fate and cell identity in a timely and robust manner results in developmental abnormalities and diseases. For example, 1 out of 100 children are born with congenital cardiac diseases, for the majority of which the causes are unknown.
To discover the regulatory programme behind heart development, the Kurian lab used embryonic stem cell-based models that recapitulate human cardiac fate decisions in a dish under chemically defined conditions. The use of human stem cell-derived models allows the team to identify human-specific attributes, which can be drastically different from other animals. The aim of this approach is to work with the most precise models closest to human biology and to minimize animal experiments.
Ribosomes as a regulatory hub to control cellular decision making
The team discovered that the competence for the future cardiac fate is preset in human embryonic stem cells (hESCs) by a specialized mRNA translation circuit controlled by the RNA binding protein RBPMS. RBPMS is recruited to active ribosomes, the molecular machine that produces proteins from mRNA. There, RBPMS controls the production of essential factors needed for the programme that triggers the stem cells to develop into heart cells.
Mechanistically, RBPMS has two functions. On the one hand, the protein interacts with components to promote the translation of mRNA to proteins; on the other hand, RBPMS selectively regulates the production of mesoderm signalling components in hESCs by binding to a specific site on the mRNA. The mesoderm is the middle layer of the three germ layers, from which the heart develops early on in embryos.
It is believed that through early contact with cardiogenic signals, the ability of stem cells to develop into future cardiac lineages is predetermined. This study shows that the RBPMS-mediated selective mRNA translation circuit approves the cellular abundance of ‘morphogen signalling infrastructure’ required for cardiac mesoderm approval in hESCs. Thus, RBPMS sets up the future cardiac competence of hESCs by programming selective mRNA translation.
“In summary, we present a model whereby the state of pluripotency is primed for differentiation into future cell lineages through specialized translation of the regulators of embryonic cell fate. Our work shows that RBPMS selectively programmes translation, i.e. the reading of mRNA and the production of proteins or mRNAs. This controls proteins and regulatory mRNAs that themselves code for important developmental regulators and are essential for deciding future cell fate,” Dr Deniz Bartsch, first author of the study, explained.
Based on their findings, the team proposes ‘translation specialization’: a regulatory mechanism that primes ribosomes to control translation in time and/or space for a set of mRNAs required for future events in response to specific stimuli or fate transitions. This allows efficient division of labour among the approximately ten million ribosomes present in each cell, which are tasked with synthesizing about two million proteins per minute, so the flow of information is streamlined and, as they show, specialized. This study, therefore, reveals a central role for translational specialization in shaping cell identity during early lineage development and proposes that ribosomes act as a unifying hub for cellular decision-making rather than a mere protein factory.
The Kurian lab investigates the regulatory principles that govern cell fate and identity during human cardiac development, homeostasis and pathomechanisms of cardiac aging. The findings from this study laid the foundations for the ERC Consolidator Grant (TRANSCEND), awarded to the Kurian lab in 2022 by the European Research Council, which aims to understand the fundamental principles by which information from the DNA is accurately and selectively translated in time and space to program the development of the human heart – and how its aberrations cause cardiac diseases.
